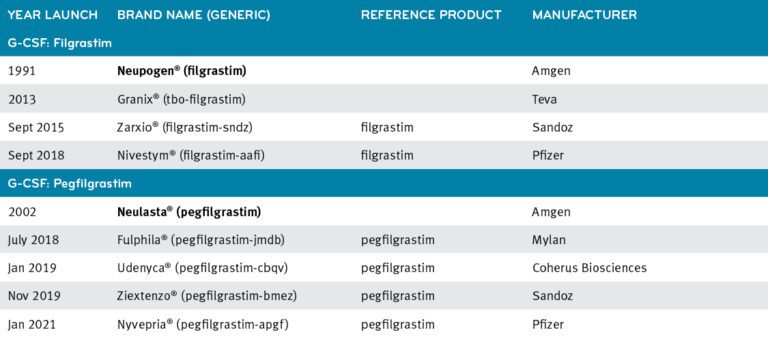
These highlights do not include all the information needed to use GRANIX safely and effectively. See full prescribing information for GRANIX.GRANIX® (tbo-filgrastim) injection, for subcutaneous use Initial U.S. Approval: 2012

Results of a Prospective Randomized, Open-Label, Noninferiority Study of Tbo-Filgrastim (Granix) versus Filgrastim (Neupogen) in Combination with Plerixafor for Autologous Stem Cell Mobilization in Patients with Multiple Myeloma and Non-Hodgkin Lymphoma -

Tevagrastim - Filgrastim 300mcg/0,5ml - Solução Injetável - Teva - ÁgilMed - Medicamentos Especiais e Nutrição Clínica