Reactive Diluents to Overcome Challenges in UV-Curable Inkjet Inks and Coatings Applications - UV+EB Technology

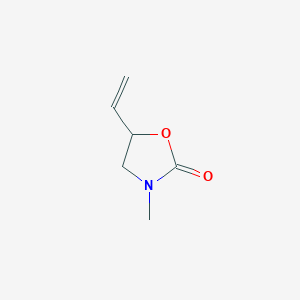
5-Methyl-3-vinyl-2-oxazolidinone–Investigations of a New Monomer for Kinetic Hydrate Inhibitor Polymers | Energy & Fuels

Recent Advances in the Synthesis and Ring‐Opening Transformations of 2‐ Oxazolidinones - Sun - 2021 - Advanced Synthesis & Catalysis - Wiley Online Library

TiCl4‐Promoted Asymmetric Aldol Reaction of Oxazolidinones and its Sulphur‐Congeners for Natural Product Synthesis - Bhamboo - 2021 - Asian Journal of Organic Chemistry - Wiley Online Library

Current Landscape and Future Perspective of Oxazolidinone Scaffolds Containing Antibacterial Drugs | Journal of Medicinal Chemistry
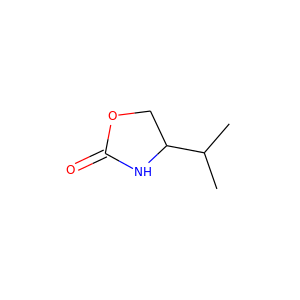
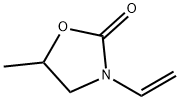
4-methyl-1,3-oxazolidin-2-one (16112-59-7) - Chemical Safety, Models, Suppliers, Regulation, and Patents - Chemchart

Lucia Veltri's research works | Università della Calabria, Rende (Università della Calabria) and other places
![3 + 1 + 1] cyclization of vinyl oxiranes with azides and CO by tandem palladium catalysis: efficient synthesis of oxazolidinones - Organic Chemistry Frontiers (RSC Publishing) DOI:10.1039/D1QO00591J 3 + 1 + 1] cyclization of vinyl oxiranes with azides and CO by tandem palladium catalysis: efficient synthesis of oxazolidinones - Organic Chemistry Frontiers (RSC Publishing) DOI:10.1039/D1QO00591J](https://pubs.rsc.org/image/article/2021/QO/d1qo00591j/d1qo00591j-s2_hi-res.gif)
3 + 1 + 1] cyclization of vinyl oxiranes with azides and CO by tandem palladium catalysis: efficient synthesis of oxazolidinones - Organic Chemistry Frontiers (RSC Publishing) DOI:10.1039/D1QO00591J